RiskGONE guidelines for Ethical Impact Assessment
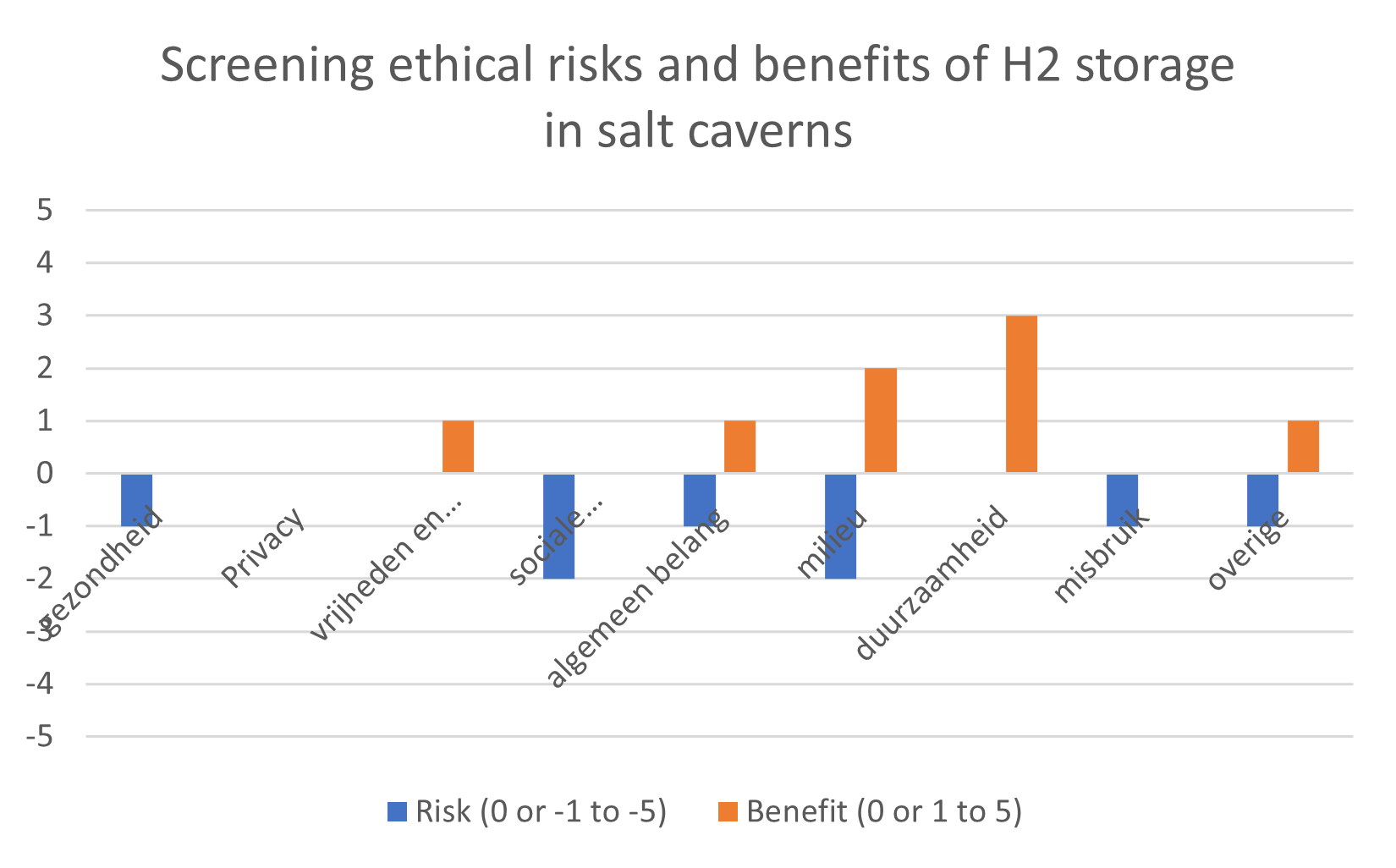
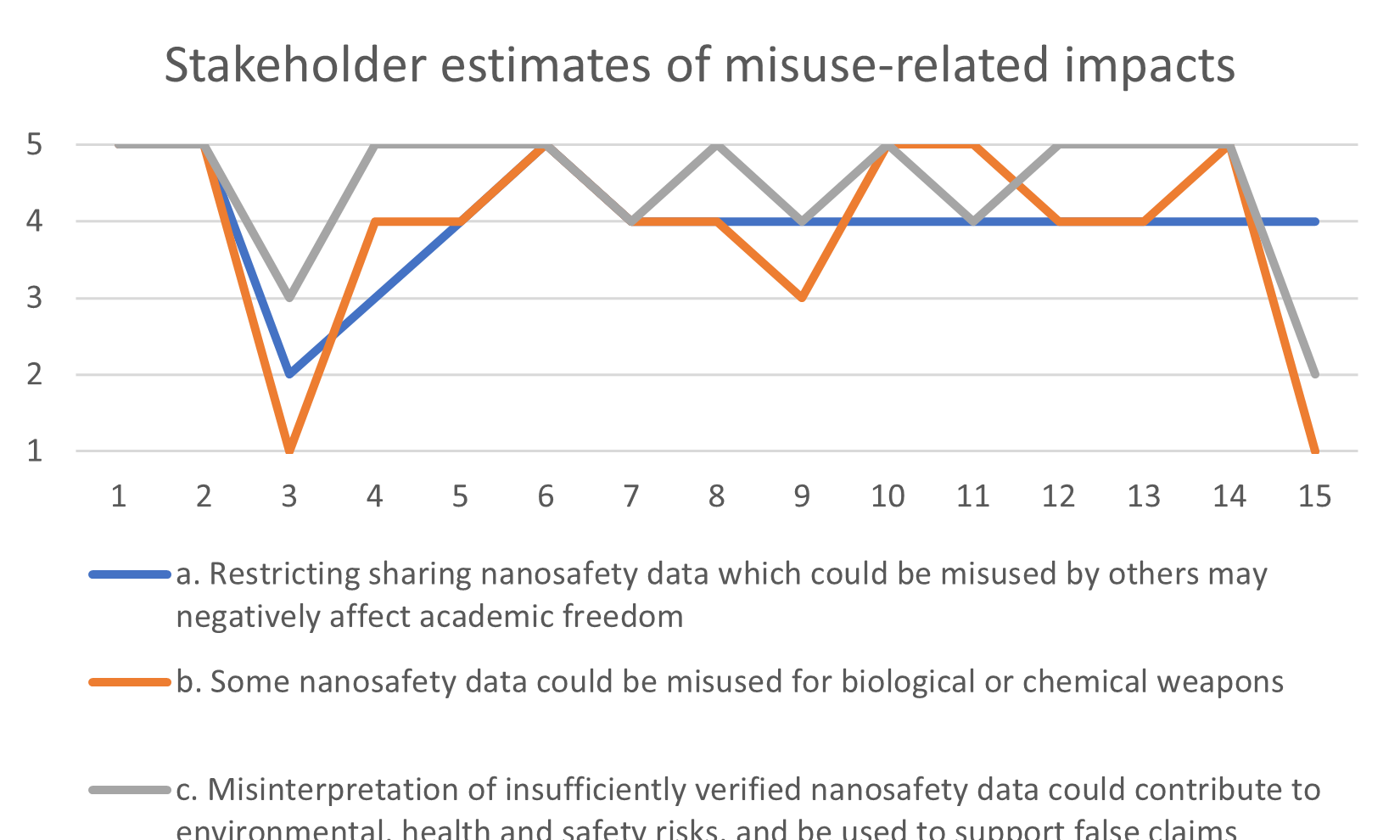
The RiskGONE project just released several reports, including Draft guidelines on Identification of regulatory and ethical risk thresholds (D3.6) by Ineke Malsch, Panagiotis Isigonis, Elena Semenzin, Evert Bouman, Maria Dusinska, Antreas Afantitis, Georgia Melagrakis. After a brief report on the work done on “Identification of regulatory and ethical risk thresholds”, guidelines are presented, supporting the user in self-assessment whether manufacturing, use or transport of a particular nanomaterial or product containing nanomaterials complies with legal requirements in the EU or specific Member States. Then, guidance on performing an Ethical Impact Assessment as described in the CEN Workshop Agreement part 2 CWA 17145-2:2017 (E) was adapted to fit the needs of members of the nano Risk Governance Council and other stakeholders interested in exploring ethical impacts of nanomaterials and nano-enabled products. These guidelines follow a six-step procedure, including screening ethical impacts, preparing an Ethical Impact Assessment plan, identifying ethical impacts, evaluating the identified ethical impacts, formulating, and implementing remedial actions, and review and audit of the Ethical Impact Assessment procedure. To illustrate the usefulness for supporting risk-benefit assessment as well as assessing only ethical risks, the guidelines for screening ethical impacts are also adapted to self-assess benefits as well as risks. Download the guidelines here.